Pre-Clinical Research & Clinical Rearch
Prior Molecules fosters product innovations through conducting best-in-class research studies in a bid to support our natural healthcare products to become an effective supplementary part of the mainstream healthcare treatments. Also, an open innovation framework is employed to form research partnerships with leading universities and research labs around the world.
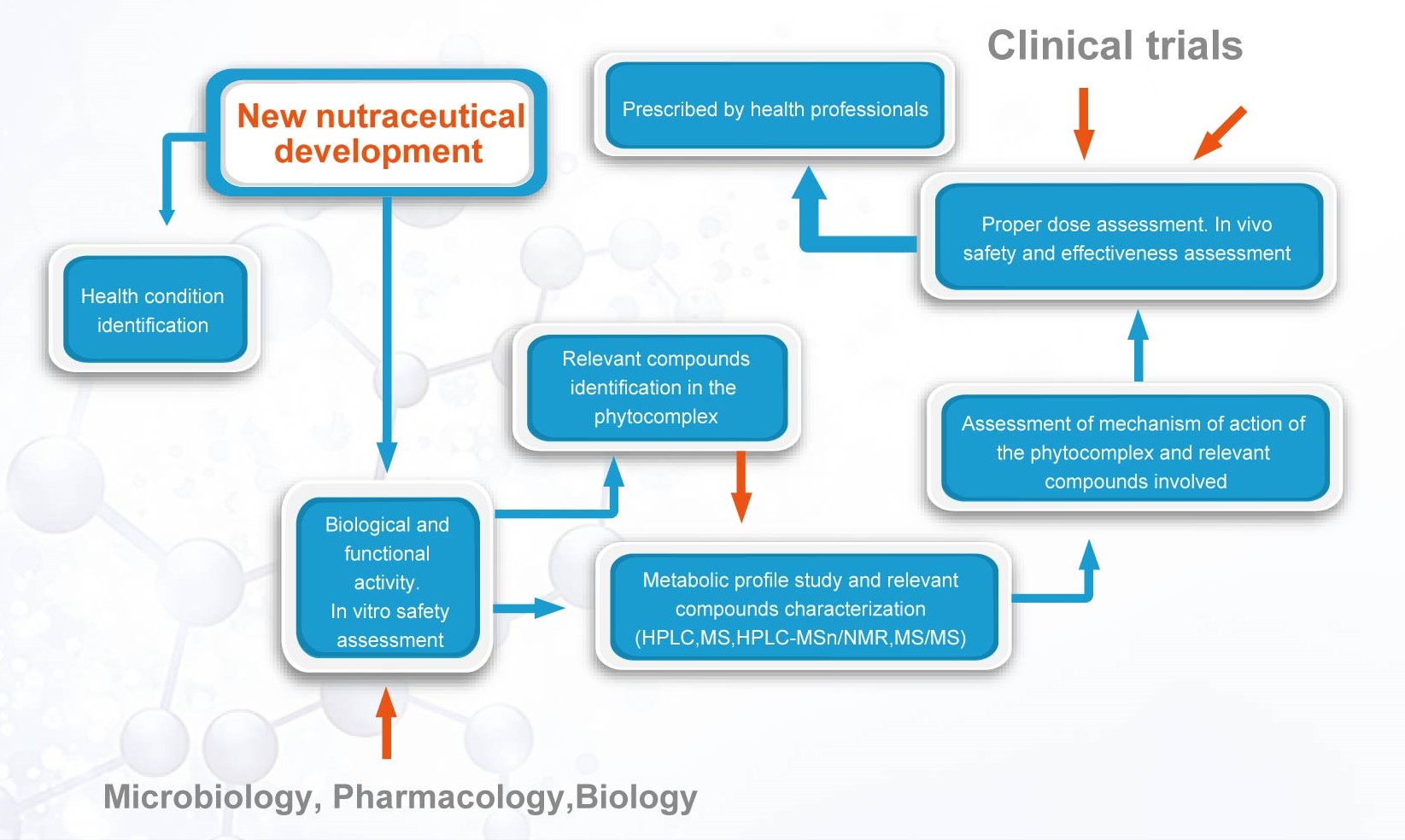
We develop and assess the feasibility of a product concept and then conduct the preclinical and clinical researches before going through the below processes:
l Source the raw materials
l Conduct pre-formulation
l Determine final formulationsand manufacturing methods
l Develop and validate analytical methods
l Conduct stability and photostabilitystudies
l Develops the Regulatory Modules
Taking the advantage of SFI’s lab scale and pilot scale manufacture capabilities, both small industrial and clinical scale batches of products can support R&D and validation of scale up production. High Pressure Liquid Chromatography (HPLC) & Ultra High Pressure Liquid Chromatography (UHPLC) capacity mean we have the analytical lab equipment to ensure every detail can be tested and then enhanced where required.
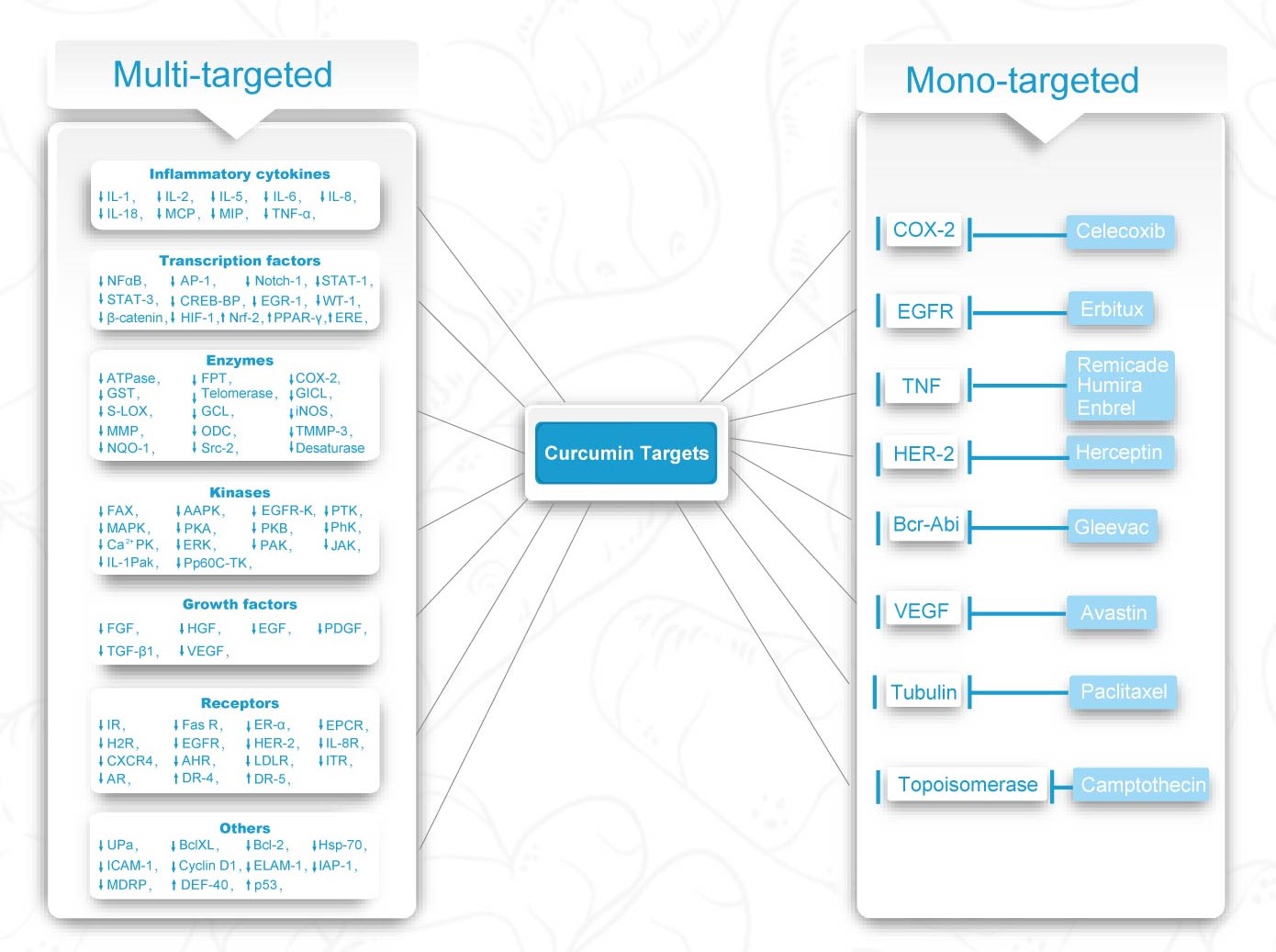
One molecule for one target V.S. Multi-molecules for multi-targets
The active principle in turmeric was identified as a group of polyphenolic compounds, namely curcumin (74-78%), demethoxycurcumin (15-18%) and bisdemethoxycurcumin (4-6%) commonly referred to as “curcumin”.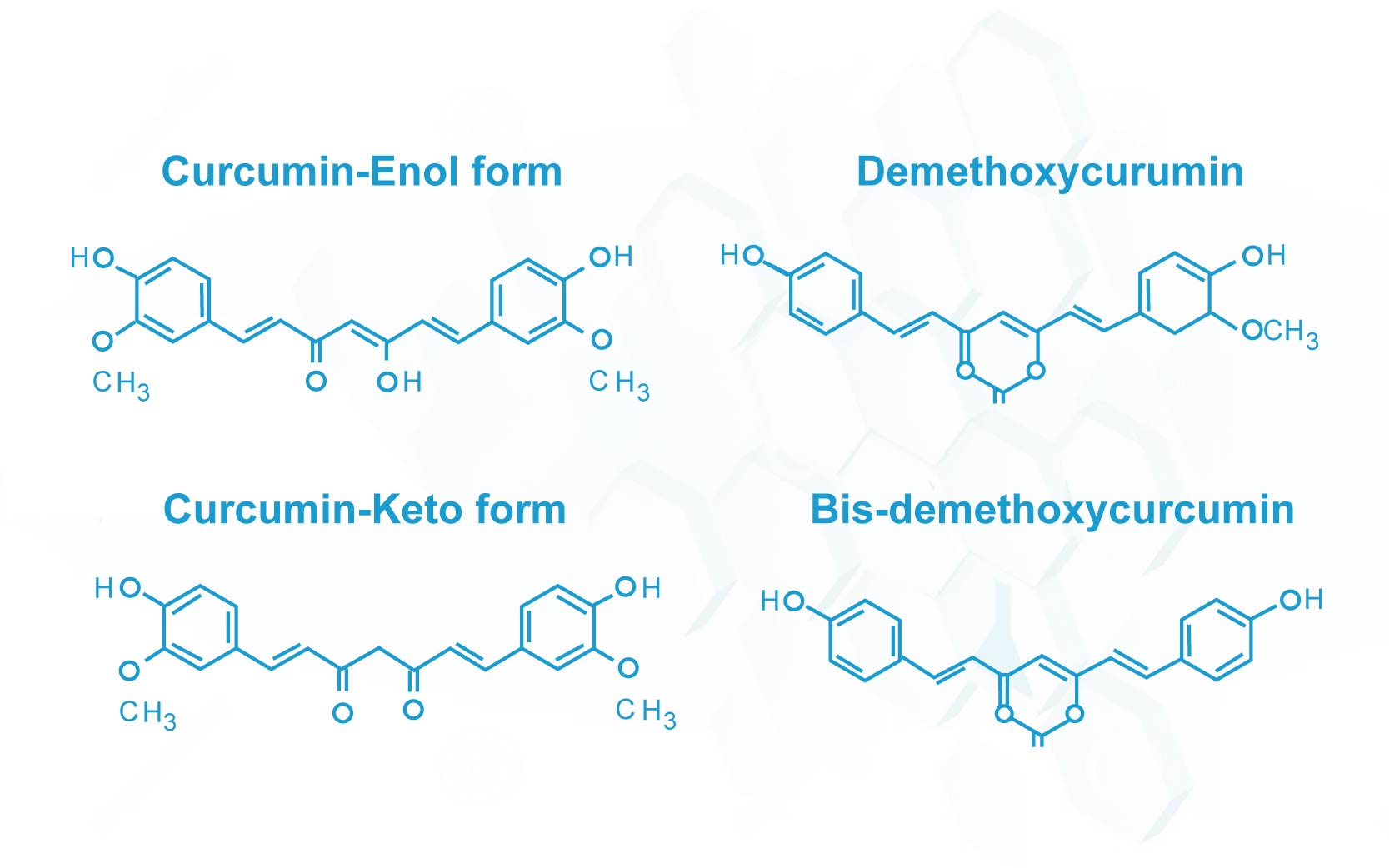
![]()
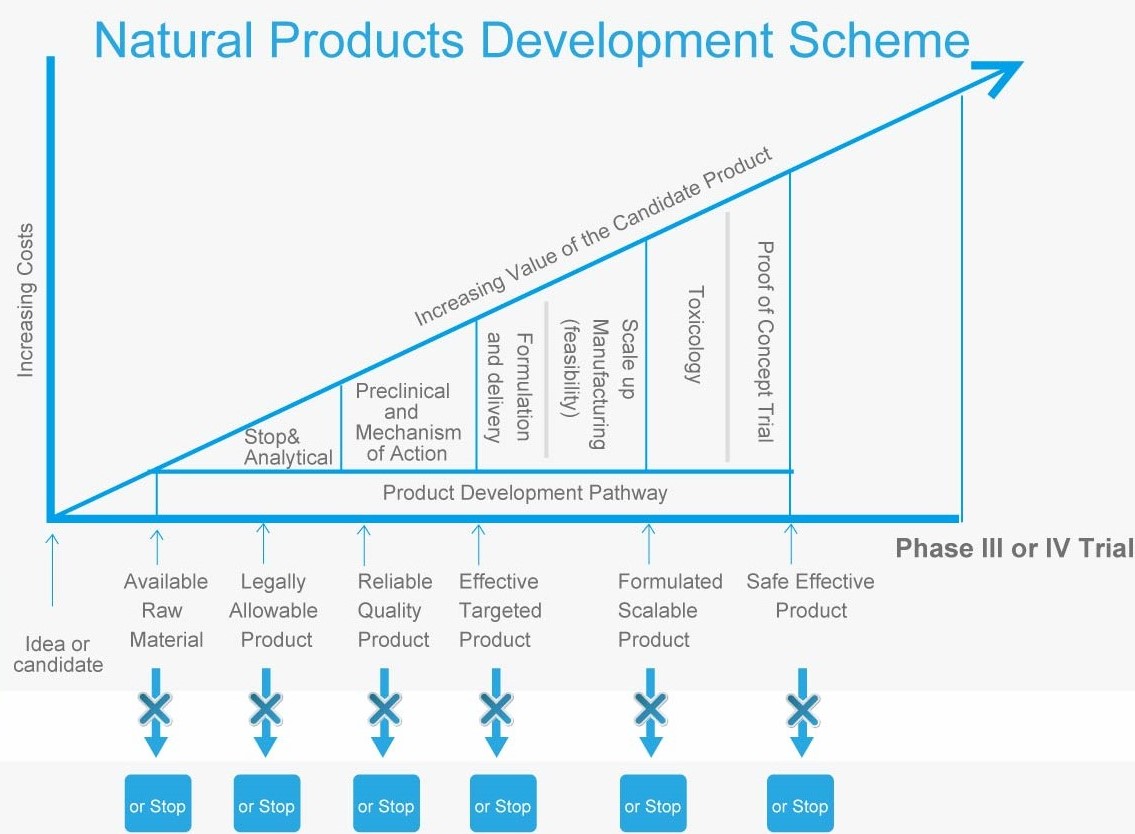
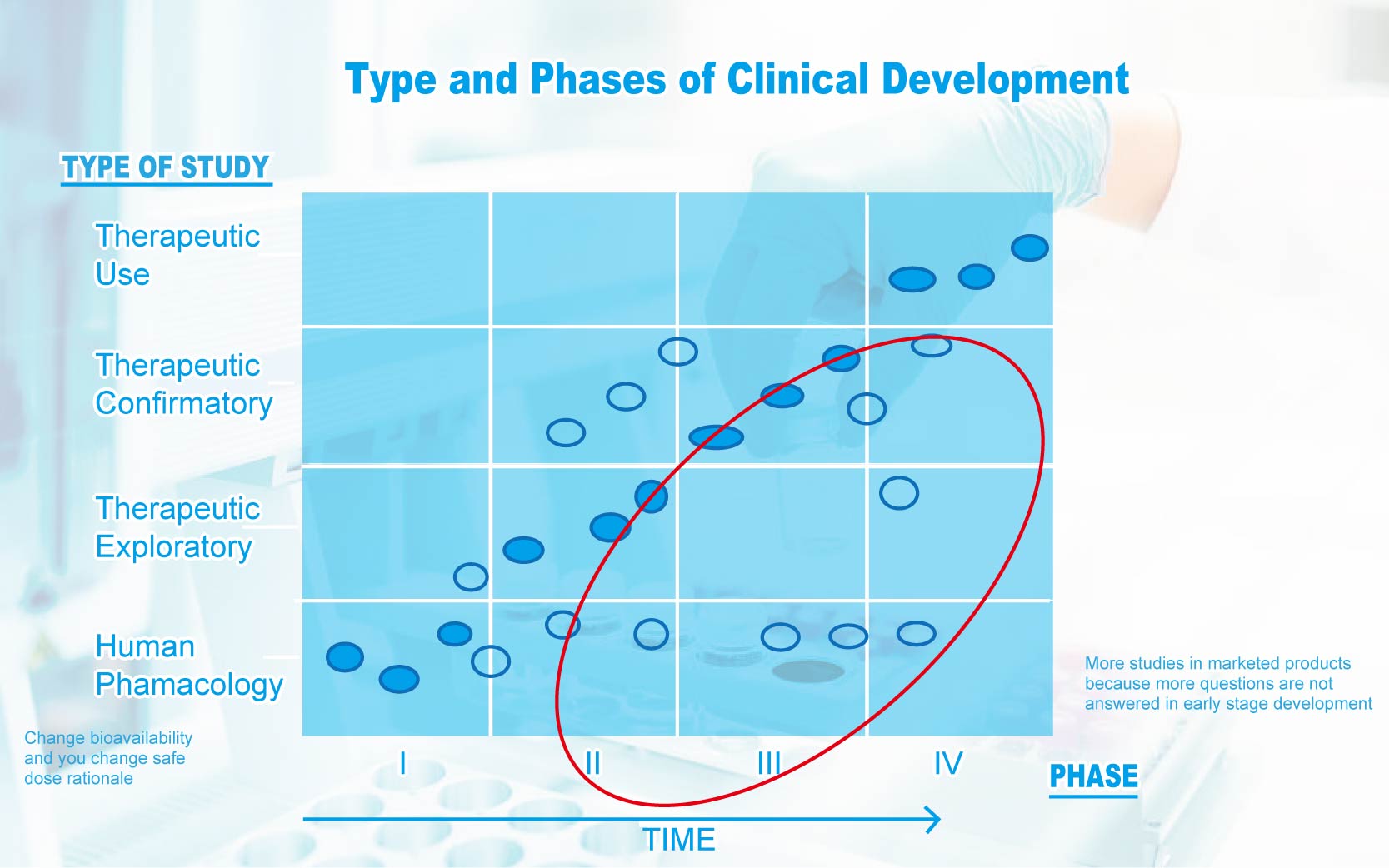
Differences from Pharmaceuticals
l Phase I and Phase II are not needed if the natural product is known to be safe
l Phase II and III are not needed if the natural product is known to be safe and the dose is known
l New data on efficacy and best model for usage can still be done at Phase IV
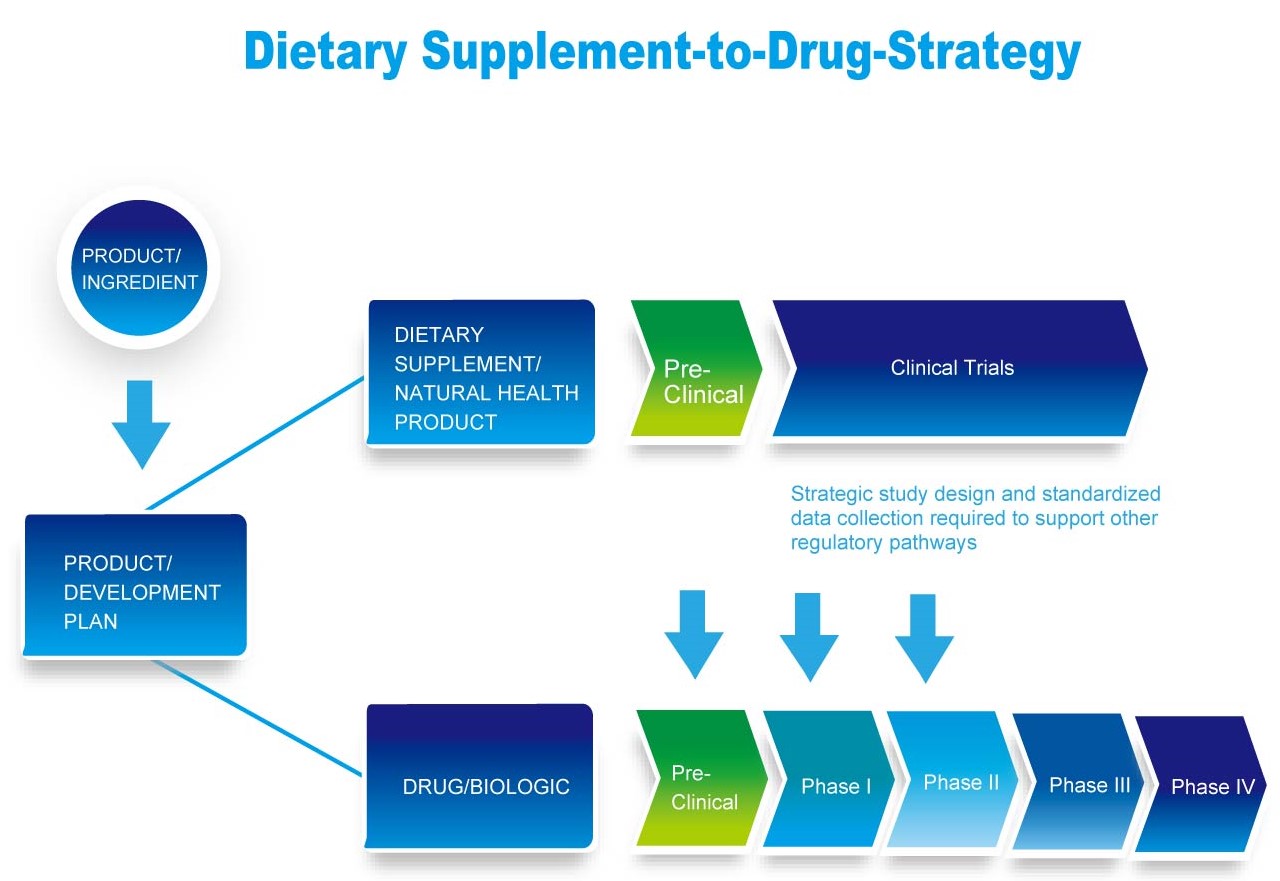
What doctors, health practitioners and patients aim at?
Efficacy
Research delivers the evidence that our products work.
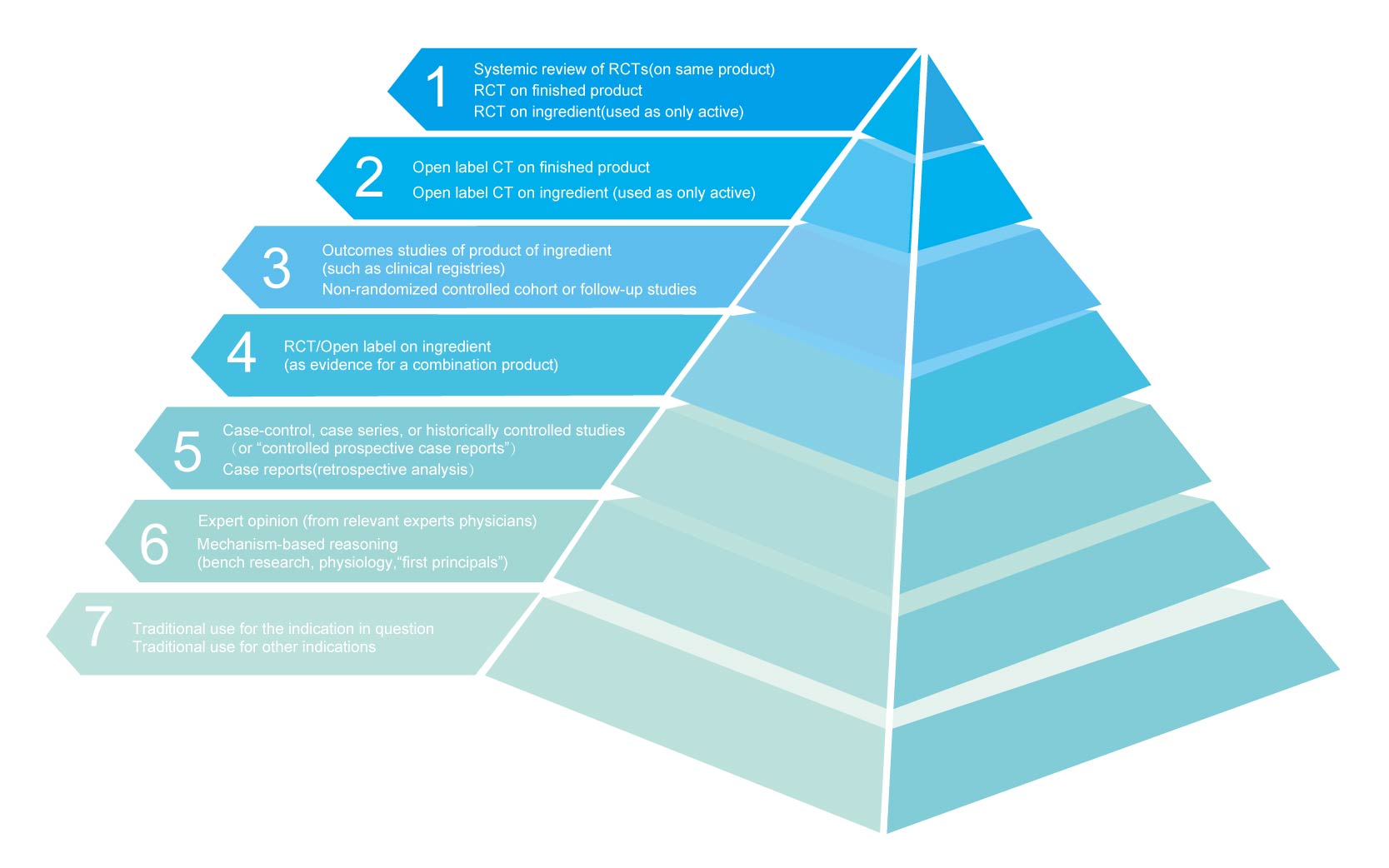
CATEGORIES
LATEST NEWS
CONTACT US
Contact:
Phone:
E-mail:
Add: